Test Drive ![]() , our AI Chatbot - get instant regulatory answers, free and live! Try Now
, our AI Chatbot - get instant regulatory answers, free and live! Try Now

Artificial Intelligence has never been more talked about or more misunderstood than it is today. The life sciences industry, heavily dependent on accuracy, compliance, and deep domain expertise, is now standing at a pivotal moment: AI is powerful, but only when implemented responsibly, built on the right foundations, and aligned with true regulatory depth.
The question that needs to be asked is: How do we move beyond AI “hype” and actually make AI work for regulatory teams?
This article breaks down the real story behind the AI Hype Cycle, the shifts happening in regulatory intelligence, and what organizations must do to stay ahead.
For years, AI captured imaginations with bold promises like automation, speed, precision, and even autonomy. But according to the 2025 Hype Cycle, GenAI has officially entered the “Trough of Disillusionment.”
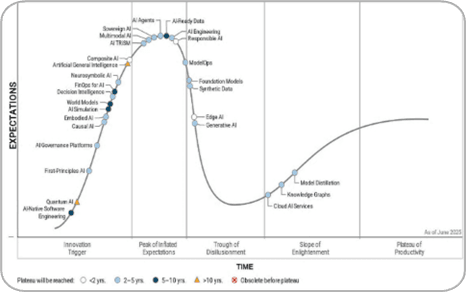
What this means in practice:
Key Insights From the Cycle
1. Responsible AI is no longer optional.
It’s now a design principle, a differentiator that builds trust and credibility, not just compliance.
2. AI-ready data is the real competitive edge.
The best models fail without high-quality, versioned, unbiased, up-to-date data.
3. The future is blended.
AI will not replace regulatory experts. Instead, the winners will blend AI + human judgment, especially near point-of-decision.
Hallucinations, bias, leaky data, copyright pitfalls, compliance issues, all originate from one place: bad data foundations.
What “AI-ready data” must include:
What this looks like at Freyr GRI
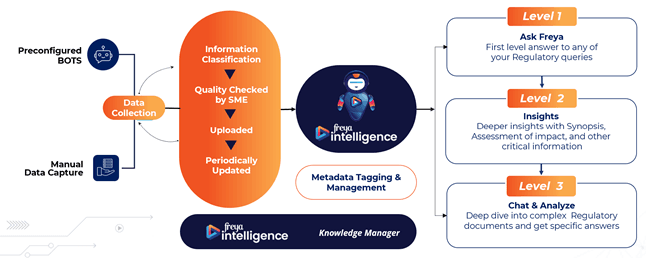
Freyr’s data workflow follows a 3-level maturity model:
Level 1: First-layer answers via “Ask freya”
Level 2: Deep-dive analytical discussions on regulatory documents
Level 3: Structured insights -> synopses, impact assessments, critical interpretations
All backed by: SME-verified information, manual + automated data capture, active metadata tagging and a dedicated knowledge manager system
AI agents promise to perceive, decide, act, and achieve goals autonomously. They’re exciting but they’re also risky, inconsistent, and often misunderstood.
| Drivers fueling their rise | Challenges leaders must consider |
| GenAI Breakthrough Multi-modal Reasoning (vision, audio, writing etc.) Adaptive Workflows for every business scenario – high expected value! (forecast, predict, plan) | Every Business Scenario is different, relevance to business is key differentiator Complex Systems, Opacity in Decision making Autonomous v Human in the loop Early Commoditization |
How Freyr Approaches AI Agents
At Freyr, we blend 14+ years of regulatory expertise with modern AI, ensuring agents are:
Our approach acknowledges a critical truth: AI is powerful, but regulatory expertise is irreplaceable.
AI success does not start at user interface, it starts underground within the architecture.
Five foundational enablers are security, privacy, modularity, integrability and innovation-readiness (MLOps, AI engineering, composite architectures)
We ensure to create a scalable ecosystem where AI can evolve without compromising compliance or usability.
To thrive in this AI era, regulatory teams must understand one reality:
It’s not the flashiest AI that wins—it’s the most responsible, data-rich, domain-deep AI.
The value of AI directly correlates with the breadth, depth, and integrity of internal + external regulatory intelligence.
AI without regulatory expertise is… automation.
AI with expertise becomes foresight.
Organizations that win:
In short:
“The companies that beat the hype start small, think long, and build on a rock-solid data foundation.”
The industry is now done with exaggerated promises. Regulators, pharma, medtech, and consumer health teams want reliable, explainable, expert-backed AI, not black boxes.
That’s exactly why we built freya.intelligence – your AI-powered Regulatory Intelligence platform that:
✔ Houses 100,000+ regulations (a repository that’s continuously updates)
✔ Covers data from 200+ markets
✔ Tracks 1,000+ new regulations weekly
✔ Contains SME-verified, continuously updated data
✔ Automates the impact assessment engine
✔ Builds Custom dashboards in weeks, not months
freya is engineered specifically for regulatory teams, combining:
Whether you’re tracking global regulatory changes, assessing impact, preparing submissions, or managing compliance complexity, freya puts clarity, speed, and foresight in your hands.
CTA: Book a Demo Now (link: https://hubs.la/Q03Lg41C0)
The AI hype cycle will continue rising and falling but regulatory leaders can’t afford turbulence. By investing in AI-ready data, foundational tech, responsible governance, and deep domain partnerships, organizations can create an enduring competitive advantage.
With Freya.Intelligence, you don’t just adopt AI, you operationalize it with precision, compliance, and global regulatory depth.
Meet the future of Regulatory Intelligence. Meet freya. Try the platform free for 14-days now!
Because regulations are multiplying faster than teams can manually track or interpret them. AI helps teams keep up with global updates, analyze impact quickly, and reduce repetitive work but only when the data and governance behind it are strong.
No. AI can accelerate searches, summarize documents, and surface risks, but the final interpretation still depends on human judgment. In regulatory work, expertise, context, and accountability cannot be fully automated.
Even the most advanced models break without clean, structured, well-governed data. AI-ready data reduces errors, prevents hallucinations, and ensures insights are actually reliable, not just fast.
Start with strong data practices, add human oversight, and ensure full transparency in how AI generates outputs. Responsible AI isn’t about saying “yes” or “no” to automation, it’s about building trust in every step of the workflow. Start with tools like freya.intelligence.
Look for depth of regulatory content, proven accuracy, human-in-the-loop validation, security, and the ability to integrate into your existing systems. A platform should make your work easier, not add another layer of complexity.


Get your regulatory dose of information delivered straight to your inbox every month!
Subscribe Now